Gases of Breathing: Oxygen
Since this is a blog about breathing it would be remiss not to discuss each of the major gases involved in breathing. These can be either taken from the air we breathe itself or produced in our own bodies as a side effect of our breathing pattern, and have a number of physiological effects.
The first of these gases I will talk about is oxygen. The stuff we all need to live. As a gas the oxygen we breathe and use to sustain our life has 2 oxygen atoms (O2) and makes up about 21% of the gas in the atmosphere, with 78% being nitrogen. To talk about oxygen and why we need it to live I first need to talk about metabolism and combustion.
For life to function it must continuously utilize energy to maintain homeostasis. Combustion is a chemical reaction where carbon-hydrogen and carbon-carbon bonds break to release a lot of energy. The freed carbon and hydrogen then bonds with oxygen to create more stable, lower energy bonds. For this reaction to take place place oxygen must be present, because even if the bonds between carbon and hydrogen or carbon are loosened up by a lot of energy (for example holding a lit match to dry tinder) unless there is a more stable configuration for them to change into (i.e. one involving bonding with oxygen) the jostled or interrupted bond just rapidly reforms.
What is means is that oxygen is what allows the energy stored in the chemical bonds of carbon and hydrogen to be freed up for use. The burning of paper is an example of rapid combustion. Biological metabolism of fuels like sugars and fats, also known as cellular respiration, is similar to combustion in that the energy of carbon-hydrogen bonds is released for use by the body by creating more stable carbon-oxygen and hydrogen-oxygen bonds.
The equation below shows one unit of sugar (C6H12O6) combining with six units of oxygen to create six units of carbon dioxide and six units of water, releasing energy in the process. These inputs and outputs are the same, whether the sugar is being “burned” in our cells or in a fire.
C6H12O6+6O2⟶6CO2+6H2O+Energy
Biological metabolism differs from combustion in that there are many more steps to the process, and the energy is released in a more controlled manner that can be transported around the cell in the form of phosphate bonds in a molecule called adenine triphosphate, or ATP.
Reactive Oxygen Species
Oxygen cleans up the excess energy that cannot be easily stored and utilized by bonding with hydrogen to create water. This step is critical because if the energy, which takes the form of free electrons, is not completely quenched via the conversion of oxygen to water what results are unstable substances called reactive oxygen species; these compounds can interfere with the structure and function of virtually any other type of organic or inorganic compound in the body in random and unforeseen ways, creating random damage to proteins and even DNA itself and giving rise to diseases or cancers.
Examples of reactive oxygen species.
Reactive oxygen species (ROS) are used as a marker of cellular stress in the body, since the application of higher energy in general, say from ultraviolet radiation or excess heat, tells the body that processes to control the presence of ROS are going awry and so a greater allostatic response is required to help prevent damage. One response is the production of antioxidants in the body. These are hyped up as healthy compounds in the food and supplement industry, and to an extent they are right in that if you have excess ROS having antioxidants can help your body neutralize those compounds.
Transport of Oxygen via Hemoglobin
Gases are much more difficult to store and transport than liquids and solids, and oxygen can’t be converted to a stable intermediate, aqueous (i.e. dissolved in water) form for easier transport without either (A) being a reactive oxygen species and therefore damaging the body or (B) being converted to water and thereby losing its ability to quench excess electrical energy from the broken carbon-carbon and carbon-hydrogen bonds. Because of this the body has several systems to transport oxygen to the tissues to replace the oxygen that is rapidly consumed by cellular respiration.
When oxygen enters our lungs it enters the blood stream in the microscopic air sacs called aveoli and binds to hemoglobin in red blood cells.
Vascularized aveoli. Oxygen enters the capillary beds and binds to hemoglobin for transport deeper into the body. Hemoglobin binds oxygen and its colour changes from bluish to blood red.
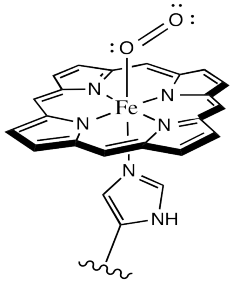
Hemoglobin is the protein that red blood cells carry around the body. There is a complex in the protein made of iron and heme, and when this complex encounters oxygen, the iron (Fe in the below diagram) bonds with oxygen. This causes the colour of hemoglobin to change from bluish to red. All blood appears red when it comes out of a cut because the hemoglobin becomes rapidly saturated with oxygen.
A heme-iron complex bonding with and holding onto oxygen.
Digital rendition of red blood cells, saturated with oxygen, flowing through arteries.
The hemoglobin holds onto oxygen until it reaches capillaries near tissues which are starved for oxygen, and then releases it there. How does hemoglobin “know” when to do this? It indirectly knows by responding to the blood chemistry changes brought about by carbon dioxide. Stay tuned for the next part in this series.